From Your local epidemiologist:
Be patient with your local pediatrician and pharmacy…
Last night, the CDC officially approved the COVID19 vaccine for 5-11 year olds. Vaccines can officially go into arms today. Unofficially, it may take a few days or a week.
***There are a lot of eligible kids***
There are 28 million 5-11 year olds in the United States. Given that 1/3 of parents are eager for vaccines, this means ~9 million kids and parents want to be in line.
And the majority of parents (63%) want to go to the pediatrician to get the vaccine. The local pharmacy (34%) and another doctor’s office (30%) comes in 2nd and 3rd.
And while many pediatrician offices will offer the vaccine, some will not. Last weekend I was on a panel with a local pediatrician who explained why. (Keep in mind that the process is state-specific. But I imagine there is a lot of overlap too). I loved her insight so I asked if she could explain in a post. This is what she said…
***During normal (non-pandemic) times…***
First, I think it’s helpful to understand what normally happens. To get a vaccine for children I place an order, pay x dollars, get a tracking number and expected date of arrival. We can store most vaccines for 6-12 months in a standard fridge. We have single dose vials, so when a child comes in for vaccines we open one vial and give them the vaccine. Because of this, very few vaccines are wasted due to the single dose vials and long shelf life.
***During abnormal (like the pandemic) times…***
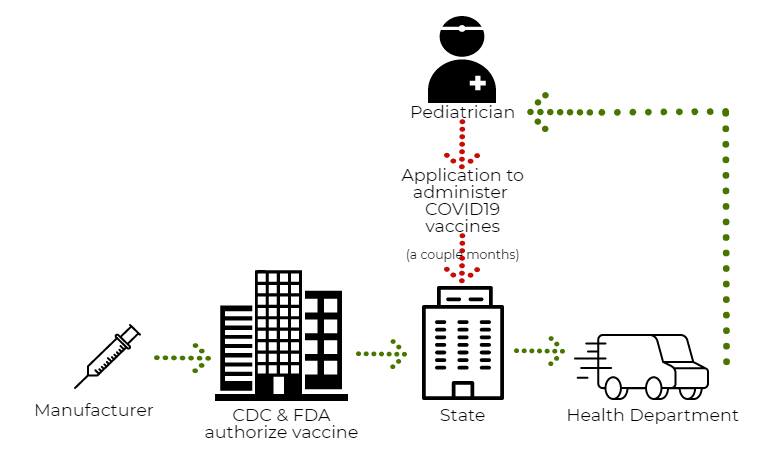
The COVID19 vaccines are under an emergency use authorization so the process is very different in many ways:
First, the storage. Currently, the mRNA vaccines are able to be stored in a regular fridge for 30 days. This is vast improvement from the beginning when they had to be stored on dry ice or in an ultra low freezer and used within 2 weeks. The new pediatric vaccines for 5-11 will be able to be stored for up to 10 weeks in the fridge. This is because Pfizer changed the buffer in the vaccine so it lasts longer at pediatrician offices. This is a welcome change.
Second, vaccines under EUA are distributed from the state. The vaccines are free as well as the supplies to give the vaccines- the needles, alcohol swabs and syringes. Interestingly gloves and bandaids are not included. In order to receive vaccines from the state, there are several applications that must be placed, approved, and processed. This process can be very laborious. For example, I need a special thermometer. Not any thermometer, but a specific one on Amazon that is, of course, now on back order. I started the application to be a COVID19 vaccinator in January 2021 and received my first COVID19 doses in May.
Then, I wait. Once I’m approved, I place an order. Over the past few months, I do not receive a tracking number. But 10-14 days after the order, I will receive a call from an unknown number telling me that they are 20 minutes away. The contracted shipping company van pulls up and the driver pulls out a cooler with a digital thermometer tracking device and transfers the vaccines to me. Supposedly, with 5-11 year olds we will get a tracking number when they ship.
Some of us anticipated the vaccine, but were thrown a wrench. The 12+ year Pfizer vaccine (30 mcg of RNA) is a purple cap that we’ve been using since December 2020. It’s been known for several months that the dose for children would be 10 mcg. So, many pediatricians (including me) ordered extra purple caps in October in anticipation of pediatric authorization. We can easily measure out 10 mcg from the purple cap vials.
But, on October 14th, it was announced that the Pfizer 5-11 vaccine would be a different size vial with a different buffer. This vaccine will have an orange cap. Pediatricians and administrators are not allowed to give 5-11 year olds doses from purple cap vials. So, the pediatricians who thought they were prepared were caught off guard with the new formulation.
Overall, because of the hoops, a lot of pediatricians opted out of giving COVID19 vaccines altogether. But your pediatrician should have a plan or recommendation on where to go, like a local pharmacy. There will be no mass vaccination sites like we had with adult vaccines.
***So, be patient with pharmacies too***
Pharmacies are distributing COVID19 primary series, COVID19 boosters, influenza vaccines, and now vaccines for 5-11 year olds. They are absolutely flooded, so offer some grace. They have also been on the frontline of the pandemic for a long time and not slowing down. Be extra vigilant and double check that they give your child the right vaccine too (orange cap).
Bottom Line: Many of us are very excited for vaccines. But you may have to wait a few days or even a week to actually get the vaccine in your child’s arm. It’s a lengthy process behind the curtain.
Love, YLE and Dr. Wolovits